Overview
Treating two patients in parallell with High-Flow Nasal Oxygen (HFNO) using the Airvo 2 system is possible due to
a. Treatment guidelines that stipulate a maximum flow should be kept as low as possible to avoid / minimise aerosol dispersion, often capped at 30 lpm.
b. The Airvo 2 system being capable of outputting 60 litres/minute (well above recommended maximum air flow)
Treating two patients in parallell with HFNO using the Airvo 2 system is feasible due to
a. Unidirectional high flow makes cross contamination unlikely
b. Using this only for cohort care with patients with the same disease makes cross contamination less significant
c. HFNO being a completely open system / circuit makes patient factors non significant for air flow dynamics
d. In the authors' limited experience the COVID-19 patients are often titrated to very similar treatments
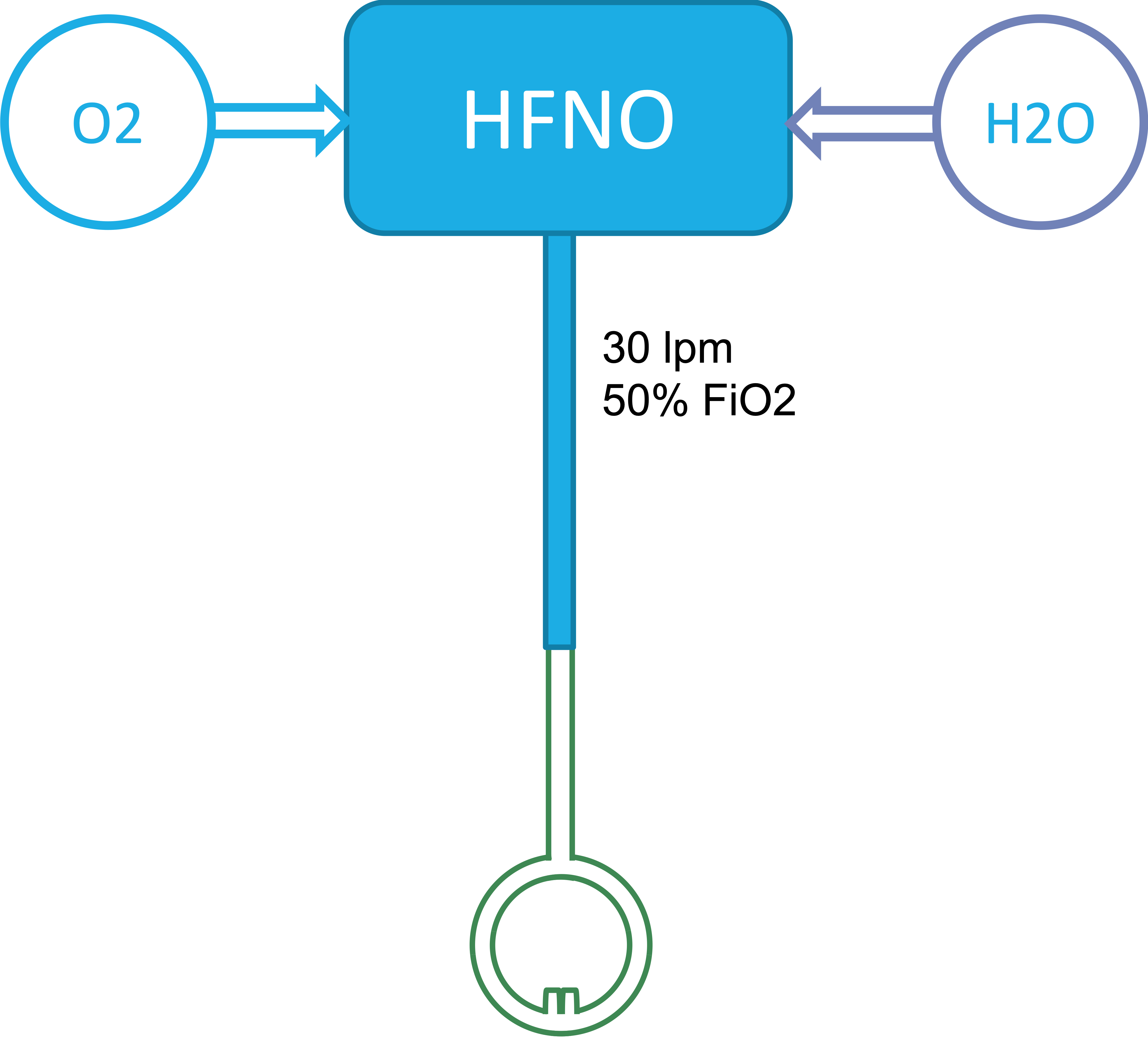
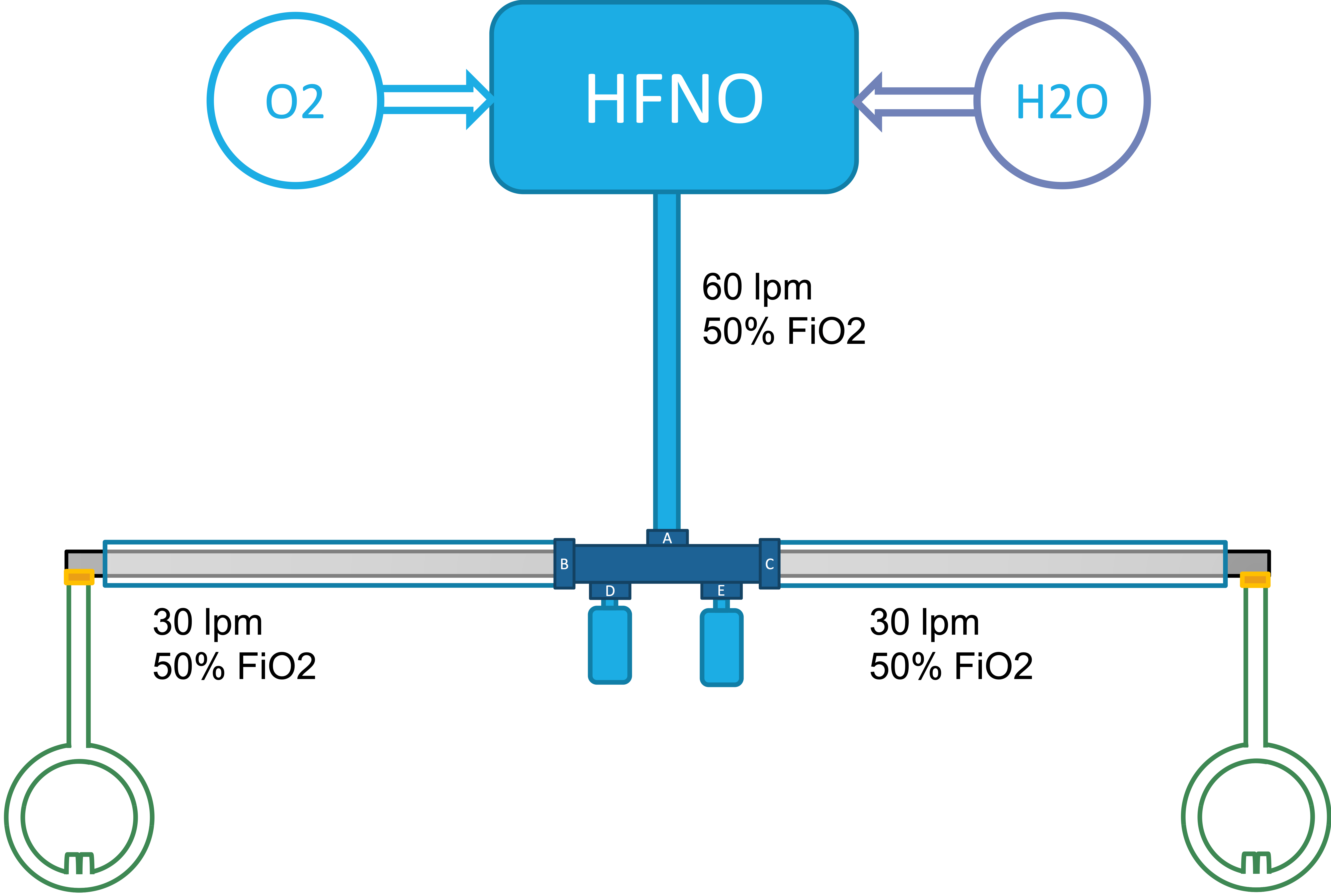
Basic Schematics
| Standard Setup | SHFNO setup |
|---|---|
 |
 |
Introduction
High-Flow Nasal Oxygen (HFNO) / High Flow Nasal Cannula (HFNC) is a staple of treatment in COVID-19, especially as many countries, regions, hospitals, and health care providers are now recommending against regularly using CPAP and Non-Invasive Ventilation (NIV)/BiPAP in these patients. This is the case in Sweden (where this project was initiated), where the Infectious Diseases Association of Sweden (IDAS, "Svenska Infektionsläkarföreningen" in Swedish) has recommended not using CPAP or NIV as well as limited upper maximums for HFNO (See below).
In the best case scenario, used in a timely and correct manner in the context of the COVID-19 pandemic, HFNO/HFNC can be used to avoid intubation and invasive ventilation. However, access to HFNO systems can be severely limited and scarcer than other equipment such as BiPAP machines.
The main system in use outside of the ICU is the Fisher & Paykel Airvo 2, often called "Optiflow", which more precisely is the special nasal cannula used with the system. The Airvo is capable of outputting 2-60 litres / minute of airflow with 21-100% FiO2 and uses wholly proprietary connectors which are not readily available and cannot be used with standardised respiratory equipment.
IDAS, as well as the Karolinska University Hospital both recommend limiting the HFNO air flow in COVID-19 patients to 30 litres / minute due to risk of aeorosolisation and the FiO2 to 50 % due to the risk of alveolar collapse. Some hospitals, like Västmanland Hospital Västerås (where this project orginated), have subsequently modified these guidelines based on clinical experience to allow higher FiO2 as well as in some cases higher air flows. Even so, the Airvo 2 is theoretically capable of supplying two patients with HFNO without impeding the maximum recommended levels of treatment according to major guidelines.
HFNO systems are well suited for split airflow, substantially more so than invasive ventilators, CPAP, or BiPAP. This is due to the open nature of the system, which causes patient factors, such as airway resistance to have minimal impact on the air flow. The unidirectionality of the air flow means that no air is shared between patients, even without added filtration. Even so, it is the authors' suggestion that the only patients eligible for treatment with Split HFNO (Hereon "SHFNO") are patients suffering from the same disease, such as COVID-19, which also means that potential cross-bcontamination across patients is less significant. We further recommend against using SHFNO for patients with confirmed secondary bacterial infections as well as for carriers of drug-resistant bacteria.
While every health care provider utilising SHFNO will need to ensure that they have medical instructions for use in place, these guidelines are provided as an example, which is subject to change and without any liability (see Legal Disclaimer). These examples are based on the medical instructions for use from Västmanland Hospital Västerås, in Sweden where the project originated.
In the limited clinical experience of the authors, COVID-19 patients treated under the recommendations above are invariably using HFNO titrated to very similar flows and FiO2. For suggestions on how to start treatment with HFNO / SHFNO please see the provided on Setup and Treatment.
To enable SHFNO we provide 3D models of
- A T-shaped connector used to connect the distal end of the Airvo connected tube (AirSpiral) with two standard 22mm CPAP/BiPAP tubes as well as two bottles used to collect condensate.
- An Adapter between the distal end of a standard 22mm CPAP/BiPAP tube and the proximal end of the Optiflow nasal cannula.
A standard SHFNO setup requires one T-shaped connector as well as two adapters.
It is our recommendation that these models be printed using high quality resin approved for medical use. Please see part 3 of this documentation, Models and Instructions for more details.
Aside from the main set provided are also models of plugs for the B/C, D/E ports as well as other adapters and an alternate T-shaped connector enabling the proprietary connectors to connect to other standardised connectors as setup might differ across hospitals.